Bacterial enzyme breaks down PFAS molecules
C-P lyase, that enables the microbe to degrade highly stable chemicals.
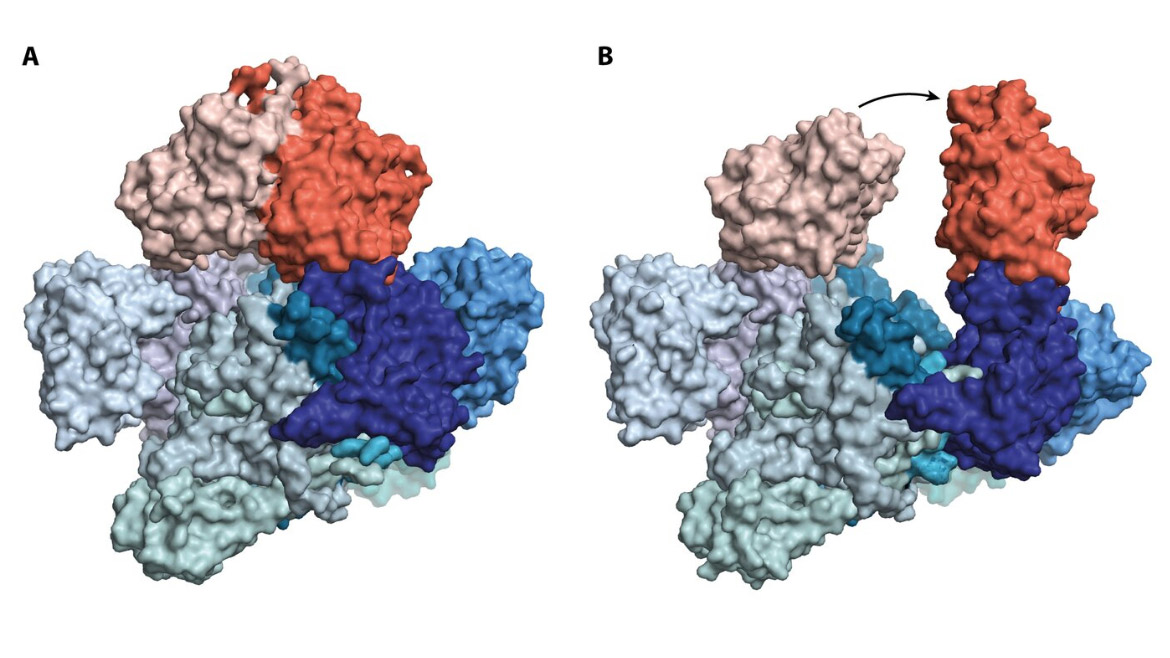
An illustration of the nutcracker enzyme in its closed (A) and open (B) states. The blue part of the enzyme contains the activity that crushes the stable substances, while the red part uses the energy from ATP to open the blue part. The enzyme binds one additional set of proteins that are used for closing (not illustrated). Credit: Søren Kirk Amstrup
Highly nondegradable chemicals such as PFAS and pesticides can have useful properties in some situations, but are extremely difficult for nature to remove afterwards. Now researchers from Aarhus University have found that certain bacteria use an enzyme that acts as a molecular nutcracker to crush the harmful substances.
All cells contain a large number of enzymes, each of which functions as a small machine that carries out a specific task. Inside E. coli bacteria, researchers have found an enzyme, C-P lyase, that enables the microbe to degrade highly stable chemicals. By rapidly freezing purified samples of the enzyme, the researchers have succeeded in capturing the molecular nutcracker in two different states that represent an open and closed form, respectively. The results show that the bacterium uses the energy from ATP, the cellular energy source, to both open and close the nutcracker.
"What we have discovered is extremely exciting," says former Ph.D. student and first author on the paper, Søren Kirk Amstrup, who now works as a researcher at the University of Copenhagen, "because it shows how nature manages to utilize modules from other systems to achieve new functions. In this case, two similar, ATP-consuming modules, which are mostly known from transport proteins, have been put together to be able to open and close the enzyme."
The new discoveries show that when the enzyme closes, the troublesome chemicals are trapped inside, where they are broken down. The C-P lyase enzyme is specific for a type of substances called phosphonates, which are used as pesticides in agriculture (e.g., RoundUp️) and as antibiotics against certain infections.
Due to their highly stable nature, this type of substances can inhibit other enzymes that stop plant growth of weeds or bacteria during an infection. But afterwards, they can be difficult to get rid of. "It is gratifying to explore systems that describe how chemicals we use in our environment are converted naturally," says Søren Amstrup, who himself comes from a farming family where RoundUp️ has been used extensively.
"It is incredibly fascinating to get an insight into the mechanism of such an enzyme, which has existed for billions of years in nature and which we are the first to see," says Professor Ditlev Egeskov Brodersen, who leads the work with C-P lyase at Aarhus University. "This enzyme has been known for over 40 years, but the mechanism has been unknown until now," he continues. "We've been working on it for 10 years and still don't understand everything that happens when the nutcracker closes, but we're well on our way and we'll probably get there."
The results, which have recently been published in the journal, Nature Communications, are expected to be useful in developing dedicated strains of bacteria that survive by breaking down the difficult substances and therefore potentially can be of great importance for the future use of pesticides in agriculture.
More information: Søren K. Amstrup et al, Structural remodelling of the carbon–phosphorus lyase machinery by a dual ABC ATPase, Nature Communications (2023). DOI: 10.1038/s41467-023-36604-y
Journal information: Nature Communications

.jpg?height=200&t=1668452997&width=200)